PRODUCT
PRODUCT
Chromic acid wastewater treatment using Sobis™
Factories that discharge hexavalent chromium must install wastewater treatment equipment and use chemicals to treat the water to reduce the level to below the specified standard before discharging the water. In 1961, we became the first company in Japan to manufacture and sell SobisTM, the most reliable, effective and economical reducing agent for treatment of wastewater containing chromium.
SobisTM is superior to other reducing agents in its: (1) high solubility, (2) small amount used, (3) small amount of sullage after treatment, and (4) low cost.
How to Use
- Hexavalent chromium wastewater is transferred from the storage tank to a reduction tank, and the pH is adjusted first with sulfuric acid to achieve a pH of 2-3. This pH of 2 to 3 provides the best conditions for treating hexavalent chromium, and is also the most economical in terms of treatment time and amount of chemicals used.
- Add SobisTM in accordance with the table below and stir to dissolve it and thereby reduce the hexavalent chromium in the yellowish-brown wastewater to convert it into trivalent chromium. When reduction is complete, the color of the wastewater changes to blue. Attaching a redox potential meter makes it easier to determine the end of reduction.
- After the reduction is completed, the wastewater is transferred to a neutralization tank, and the wastewater is neutralized to pH 7.5 to 8.0 using caustic soda, etc.
- It is transfered from the neutralization tank to a settling tank. Neutralization converts trivalent chromium to insoluble chromium hydroxide, which floats and then precipitates over time.
- After precipitation is completed, the supernatant liquid is drained.
- The precipitate is removed from the tank in a timely manner, dehydrated, and disposed of as waste.
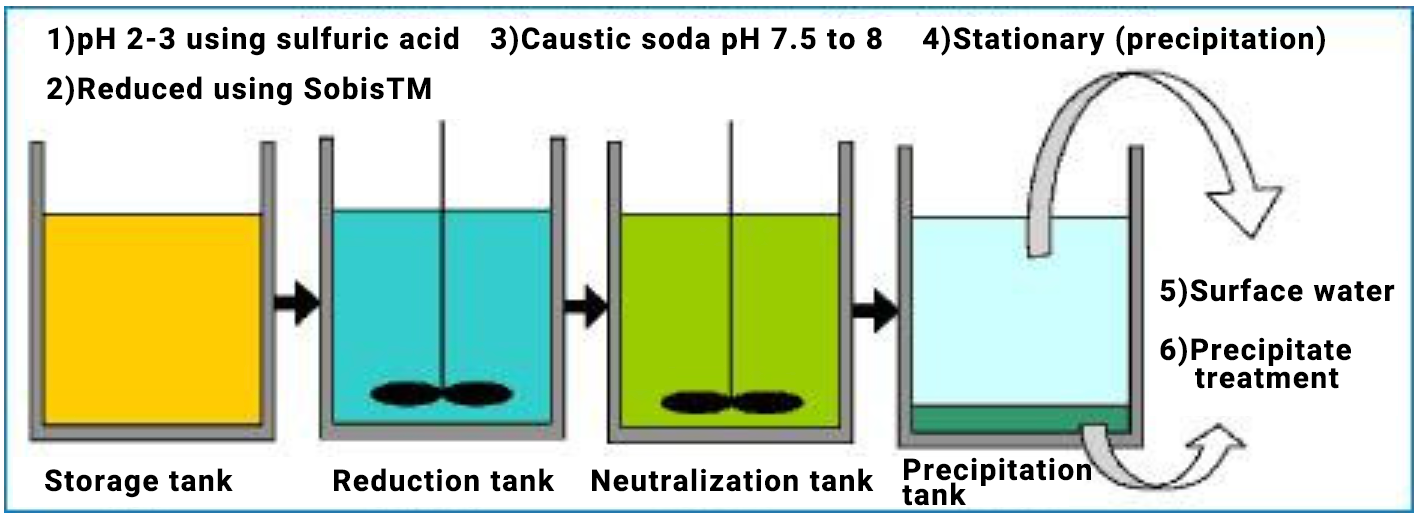
Table: Theoretical amounts of chemicals required (kg) to treat 1 (kg) object
| Product name/object to be treated | Sulfuric acid (used before treatment) H2SO4 |
SobisTM Na2S2O5 |
Caustic soda (used after treatment) NaOH |
Amount of chromium hydroxide precipitate produced after treatment (kg) |
|---|---|---|---|---|
| Chromic anhydride | 0.74 | 1.43(*) | 1.20 | 1.20 |
| Sodium dichromate | 0.94 | 1.09 | 0.92 | 0.92 |
| Chromium | 1.42 | 2.70 | 2.30 | 2.30 |
The concentration and quantity of the object to be treated are required in order to calculate the theoretical quantity. The theoretical amount of SobisTM can be obtained by substituting these values into the following equation.
[Target concentration (%)] x [Treatment volume (L)] x [Value in the above table] ÷100 = [Theoretical amount (kg)]
*Calculation example For example, when 300L of wastewater containing 2% chromic anhydride is treated with SobisTM, substitute the following into the above equation
2 × 300 × 1.43 ÷ 100 = 8.58
8.58 kg is the theoretical amount of SobisTM.
The above is a theoretical amount, so more may be needed in practice. Finally, please measure the object to be treated to confirm the endpoint.

